b) A voltaic cell is up 25°C with the half-cells, AIAP (0.001 M) and NIIN 0.50 M Write the equation the reaction that occurs when the cell generates an electric current and
A voltaic cell is set up at 25°C with the following half-cells, Al^3+ (0.001 M) and Ni^2+ (0.50 M). - Sarthaks eConnect | Largest Online Education Community

SOLVED: A voltaic cell using Pb/Pb and Ni/Ni half-cells is set up at standard conditions, and each compartment has a volume of 355 mL. What is the cell potential, in V, after

Chapter 20 - Electron Transfer Reactions Objectives: 1. Carry out balancing of redox reactions in acidic or basic solutions; 2. Recall the parts of a basic. - ppt download
A voltaic cell is setup at 25°C with the half cells Ag^+ (0.001 M) Ag and Cu^2+ (0.10 M) Cu. What should be its cell potential ? - Sarthaks eConnect | Largest Online Education Community

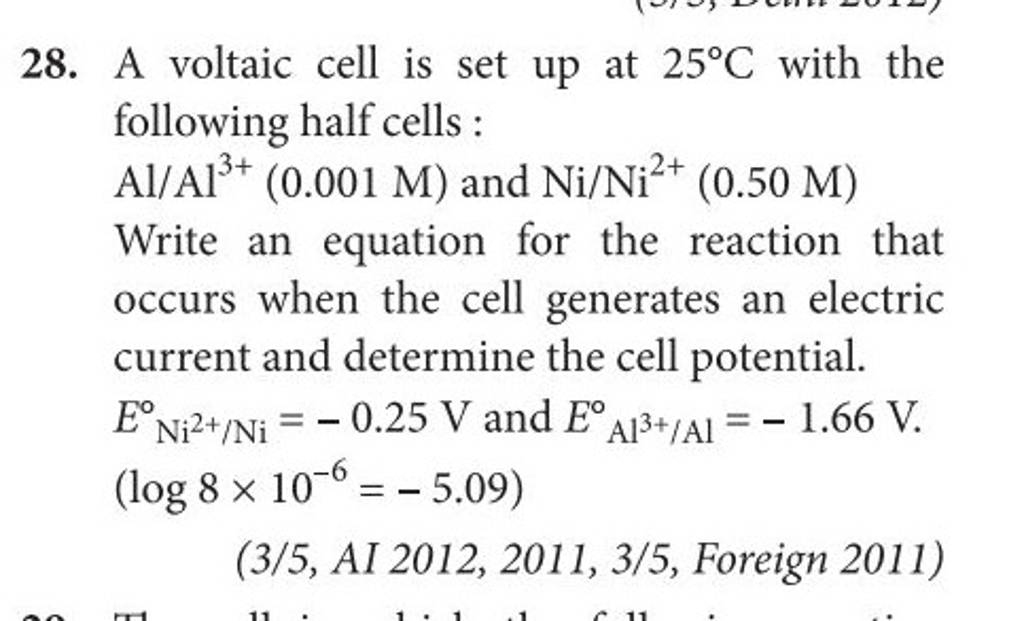
3). A voltaic cell is up 25°C with the following half cells : A1+ (0.001 M) and Ni+2 (0.50 M) Write the cell reaction and calculate the cell potential. (Given : Ex+3/4 = -
A voltaic cell is set up at 25°C with the following half cells. Al | Al^3+ (0.0010 M) and Ni^2+ (0.50 M) | Ni - Sarthaks eConnect | Largest Online Education Community

A silver concentration cell is set up at 25 degrees C as shown below. The AgCl(s) is in excess in the left compartment. Label the anode and cathode, and describe the direction
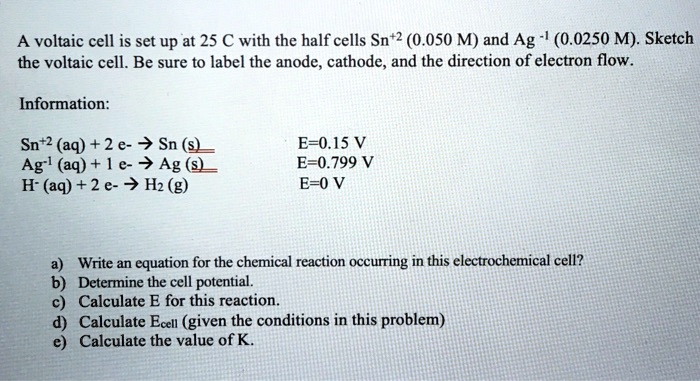
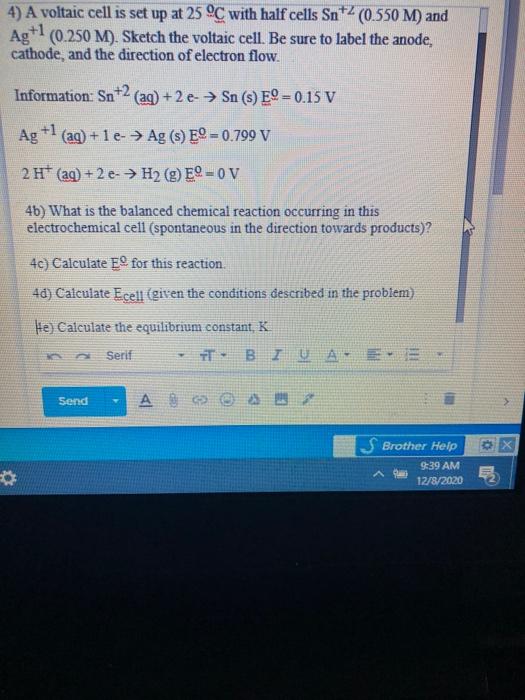
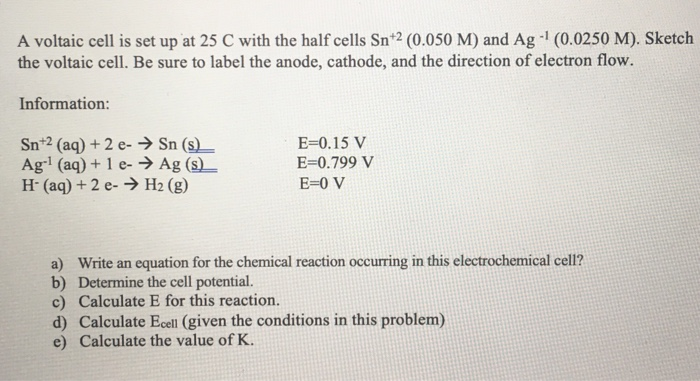
SOLVED: A voltaic cell is set up at 25 C with the half cells Sn+2 (0.050 M) and Ag (0.0250 M). Sketch the voltaic cell. Be sure t0 label the anode, cathode

pls answer the whole question 14 A voltaic cell is set up at 25 C with the following - Chemistry - Electrochemistry - 13701485 | Meritnation.com

3). A voltaic cell is up 25°C with the following half cells : A1+ (0.001 M) and Ni+2 (0.50 M) Write the cell reaction and calculate the cell potential. (Given : Ex+3/4 = -
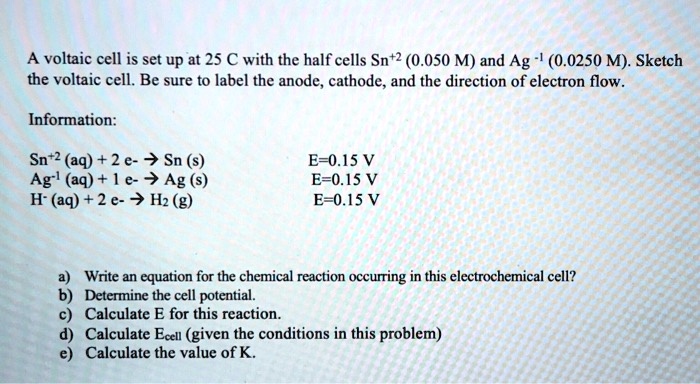
SOLVED: A voltaic cell is set up at 25 € with the half cells Sn+2 (0.050 M) and Ag (0.0250 M): Sketch the voltaic cell. Be sure to label the anode, cathode

SOLVED: A voltaic cell using Pb²⠺/Pb and Ni²⠺/Ni half-cells is set up at standard conditions, and each compartment has a volume of 355 mL. What is the cell potential after

b) A voltaic cell is up 25°C with the half-cells, AIAP (0.001 M) and NIIN 0.50 M Write the equation the reaction that occurs when the cell generates an electric current and
![SOLVED: A voltaic cell using Cu²⁺/Cu and Al³⁺/Al half-cells is set up at standard conditions, and each compartment has a volume of 225 mL. What is the [Al³⁺] after the cell has SOLVED: A voltaic cell using Cu²⁺/Cu and Al³⁺/Al half-cells is set up at standard conditions, and each compartment has a volume of 225 mL. What is the [Al³⁺] after the cell has](https://cdn.numerade.com/ask_previews/7432e1ce-8659-463e-96db-09a10402fec1_large.jpg)
SOLVED: A voltaic cell using Cu²⁺/Cu and Al³⁺/Al half-cells is set up at standard conditions, and each compartment has a volume of 225 mL. What is the [Al³⁺] after the cell has

M UZ2 = 200.6 g mol-' b) A voltaic cell is up 25 °C with the following half- cells A1S- (0.001 M) and Ni2+ (0.50 M). Write an equation the reaction that occurs

A voltaic cell is set-up 25°C with the following half-cells. Ag (0.001 M) | Ag and Cu²+(0.10 M) | Cu What would be the voltage of this cell? [Given, Ecell = 0.46
A voltaic cell is set up at 25°C with the following half-cells, Al^3+ (0.001 M) and Ni^2+ (0.50 M). - Sarthaks eConnect | Largest Online Education Community